FABHALTA RESULTS
FABHALTA was studied in adults with IgAN
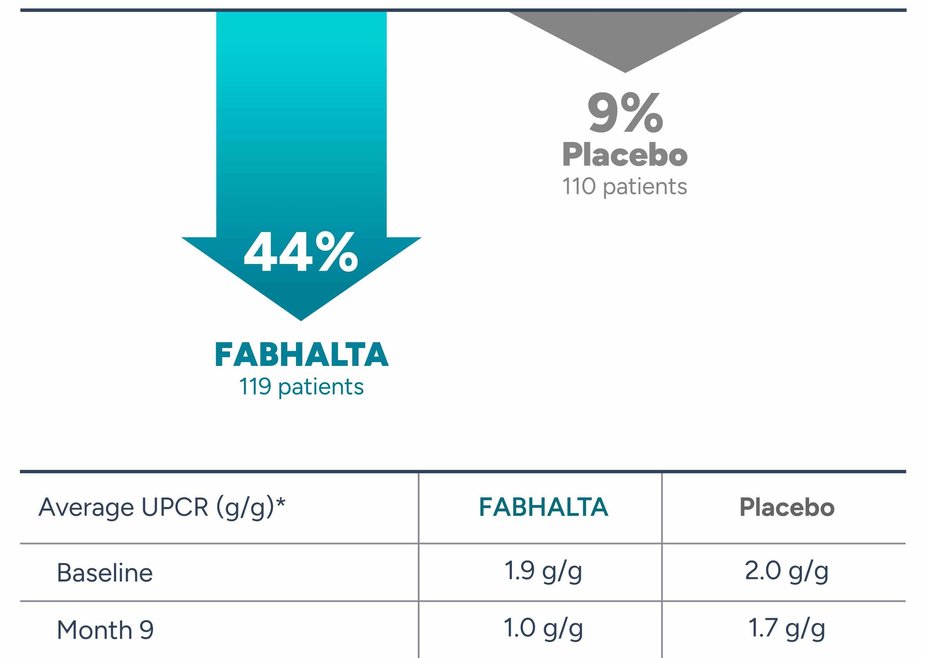
The APPLAUSE-IgAN trial is a phase 3, placebo-controlled clinical study for adults with primary IgAN that studied the percentage reduction in urine protein-to-creatinine ratio, or UPCR, a test used to measure protein in urine. In this study, urine samples were collected over 24 hours, with results from the start of the study compared against those after 9 months.
Who was studied?
Patients with biopsy-proven IgAN with elevated proteinuria (UPCR ≥1g/g) who were on a stable dose of maximally tolerated blood pressure medications (ACEi/ARB) with or without other background therapies before and during the study.
How was the study done?
Efficacy results were analyzed after the first 125 patients taking FABHALTA (200 mg twice daily) and 125 patients taking placebo (sugar pill) received 9 months of study drug.
FABHALTA substantially reduced proteinuria in adults with primary IgAN at risk of their disease worsening quickly
Primary study objective
FABHALTA was shown to substantially reduce proteinuria levels at 9 months compared to placebo (sugar pill).
FABHALTA is used to reduce proteinuria in adults with primary IgAN at risk of their disease worsening quickly.
Proteinuria reduction at 9 months*
*Percent reduction was calculated by comparing average proteinuria levels at the start of the study and at 9 Months; Results for patients requiring rescue treatments for IgAN were assumed to relate to disease worsening. As of Month 9, 7 (5.6%) patients in the placebo (sugar pill) arm and 0 patients in the FABHALTA arm received rescue treatment for IgAN.
Did you know?
Increasing levels of proteinuria over time is an important indicator that your IgAN may be getting worse.
Ask your doctor how FABHALTA can help reduce proteinuria.