

The long-term effect of FABHALTA was studied through 2 years
The effect of FABHALTA on increasing hemoglobin (Hb) by ≥2 g/dL was studied
These data are presented for observation only. It is unknown if the following results were due to FABHALTA. We cannot make conclusions from these data, but they are useful to help guide future research.
Percentage of people who had increased Hb levels by ≥2 g/dL at 2 years

*Percentage at 2 years is based on the number of people with Hb results at that time point.
The effect of FABHALTA on reaching hemoglobin (Hb) levels of ≥12 g/dL was studied
These data are presented for observation only. It is unknown if the following results were due to FABHALTA. We cannot make conclusions from these data, but they are useful to help guide future research.
Percentage of people who reached Hb levels of ≥12 g/dL at 2 years

Normal Hb levels vary but are generally between 12-16 g/dL for women and 13-18 g/dL for men.
*Percentage at 2 years is based on the number of people with Hb results at that time point.
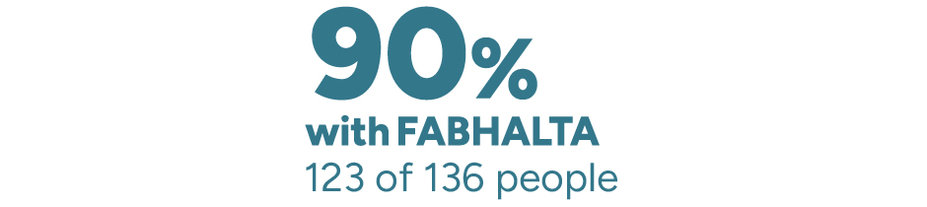
The need for red blood cell (RBC) transfusions was studied
These data are presented for observation only. It is unknown if the following results were due to FABHALTA. We cannot make conclusions from these data, but they are useful to help guide future research.
Percentage of people who didn’t need RBC transfusions through 2 years
Fatigue was measured using a questionnaire
Before the study began and at various points during the study, people rated their fatigue using the FACIT-Fatigue scale, a 13-item questionnaire used to measure fatigue and its impact on daily activities and function.
FACIT-Fatigue scores range from 0 to 52

*The average FACIT (Functional Assessment of Chronic Illness Therapy)-Fatigue score for the general population was determined in a study of 1010 adults in the United States in 2002 and 2426 adults in Germany in 2018.
Fatigue was studied with FABHALTA
Data from this analysis describe how people reported their fatigue. There are important limitations to the data presented below that you should consider:
Because people knew whether they were on FABHALTA, they may have under- or over-estimated their fatigue
In the APPLY Study: While on SOLIRIS® or ULTOMIRIS® and before starting FABHALTA, about half the people rated their fatigue as “a little bit” and “not at all” for 10 of the 13 items in the questionnaire. Because of the small number of people in the study (97), the low level of fatigue reported before taking FABHALTA, and since people knew which treatment they were on, it is unknown if these results were due to FABHALTA and no conclusions can be made about the effect of FABHALTA on fatigue. No conclusions or comparisons between FABHALTA and SOLIRIS® or ULTOMIRIS® can be made based on these data
In the APPOINT Study: Because of the small number of people in the trial (40) and the fact that people knew the medicine they were taking, it is unknown if these results were due to FABHALTA and no conclusions can be made about the effect of FABHALTA on fatigue
Average change in FACIT-Fatigue score at 2 years compared to the start of the study

*Only those people who had scores at the start of the study and during the study are included.
Hear from people who went from “fine” to FABHALTA
Use our conversation starter to talk to your doctor about FABHALTA
