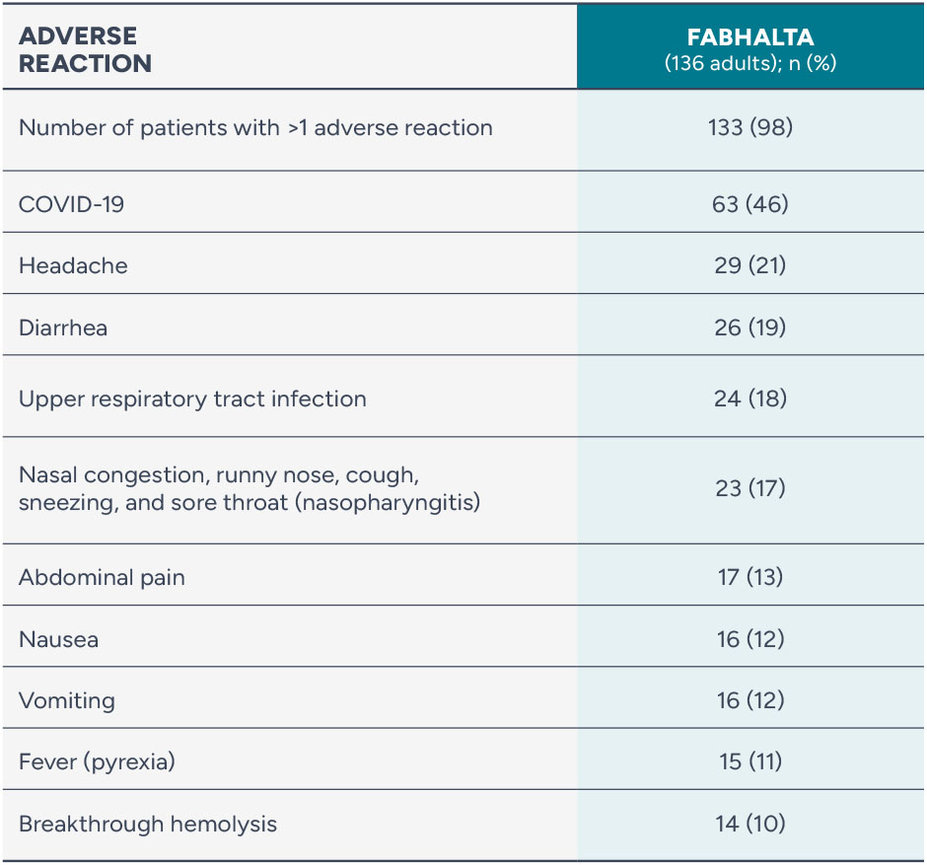
The long-term safety of FABHALTA was studied through 2 years
Adverse reactions reported in ≥10% of people
Four people discontinued FABHALTA during the follow-up study: 2 due to death, 1 due to an adverse reaction, and 1 due to physician decision.
ULTOMIRIS (ravulizumab-cwvz) and SOLIRIS (eculizumab) are registered trademarks of Alexion Pharmaceuticals, Inc.