FABHALTA was better at improving Hb levels than SOLIRIS® or ULTOMIRIS®
The study was primarily set up to understand the percentage of people who had increased Hb levels by ≥2 g/dL without a need for red blood cell (RBC) transfusions.
Percentage of people who had increased Hb levels by ≥2 g/dL without a need for RBC transfusions
The average Hb level at the start of the study was 8.9 g/dL for both groups, which is below normal Hb levels
Normal Hb levels vary but are generally between 12-16 g/dL for women and 13-18 g/dL for men
FABHALTA helped normalize hemoglobin (Hb) levels to ≥12 g/dL, an important goal in PNH treatment
The study was primarily set up to understand the percentage of people who had normalized Hb levels of ≥12 g/dL without a need for RBC transfusions.
Percentage of people who had normalized Hb levels of ≥12 g/dL without a need for RBC transfusions
The average Hb level at the start of the study was 8.9 g/dL for both groups, which is below normal Hb levels
The term “normalized Hb” refers to achieving Hb levels of ≥12 g/dL
Normal Hb levels vary but are generally between 12-16 g/dL for women and 13-18 g/dL for men
Increased Hb levels may help improve PNH symptoms due to anemia
People had increased Hb levels after switching to FABHALTA from SOLIRIS® or ULTOMIRIS®
These data are presented for observation only. It is unknown if the following results were due to FABHALTA. We cannot make conclusions from these data, but they are useful to help guide future research.
The study was primarily set up to determine changes in Hb levels from the start of the study.
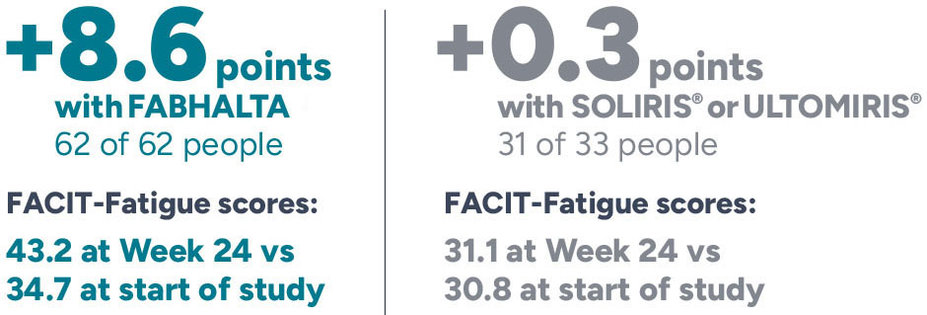
Average change in Hb from the start of study
*Average was assessed between Weeks 18 and 24. Hb levels within 30 days of a red blood cell transfusion were excluded.
ULTOMIRIS (ravulizumab-cwvz) and SOLIRIS (eculizumab) are registered trademarks of Alexion Pharmaceuticals, Inc.