Safety profile of FABHALTA
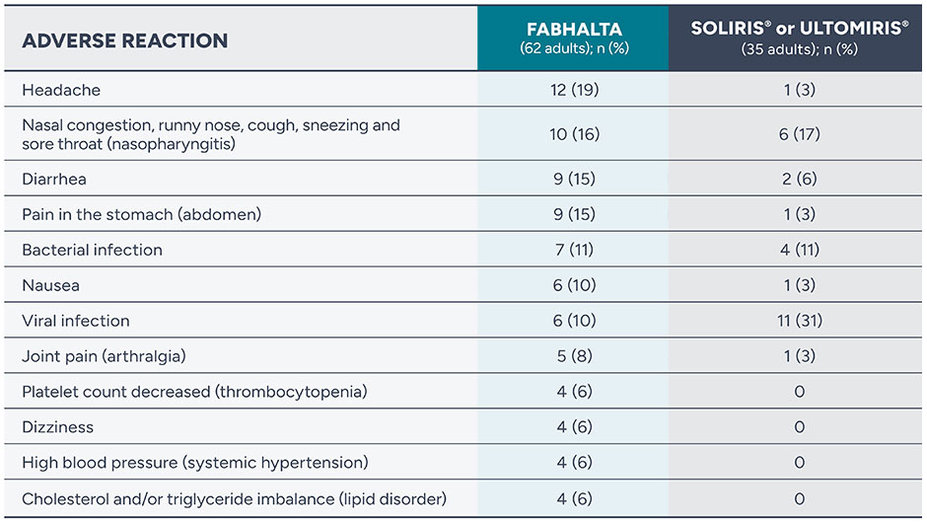
Adverse reactions reported in more than 5% of adults with PNH treated with FABHALTA during the initial study period (Weeks 0 to 24)
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of FABHALTA.
Serious adverse reactions (kidney infection, urinary tract infection, and COVID-19) were reported in two people (3%) with PNH receiving FABHALTA
FABHALTA may increase your cholesterol and triglycerides and your health care provider will do blood tests to check these periodically during treatment
Rash was reported in two people (3%) taking FABHALTA
Serious adverse reactions (deep skin infection caused by bacteria and low platelet levels) were reported in 2% of people on FABHALTA (2 of 96)
Adverse reactions that occurred in more than 5% of people were viral infection (22.9%), bacterial infection (14.6%, including 6.3% of kidney and urinary infection), nasal congestion, runny nose, cough, sneezing, and sore throat (11.5%), diarrhea (5.2%), headache (5.2%), nausea (5.2%), and low platelet levels (5.2%)
Throughout the 48 weeks of this study, no person discontinued any of the treatments due to adverse reactions. Two people in the study discontinued FABHALTA due to pregnancy.
Safety profile of FABHALTA
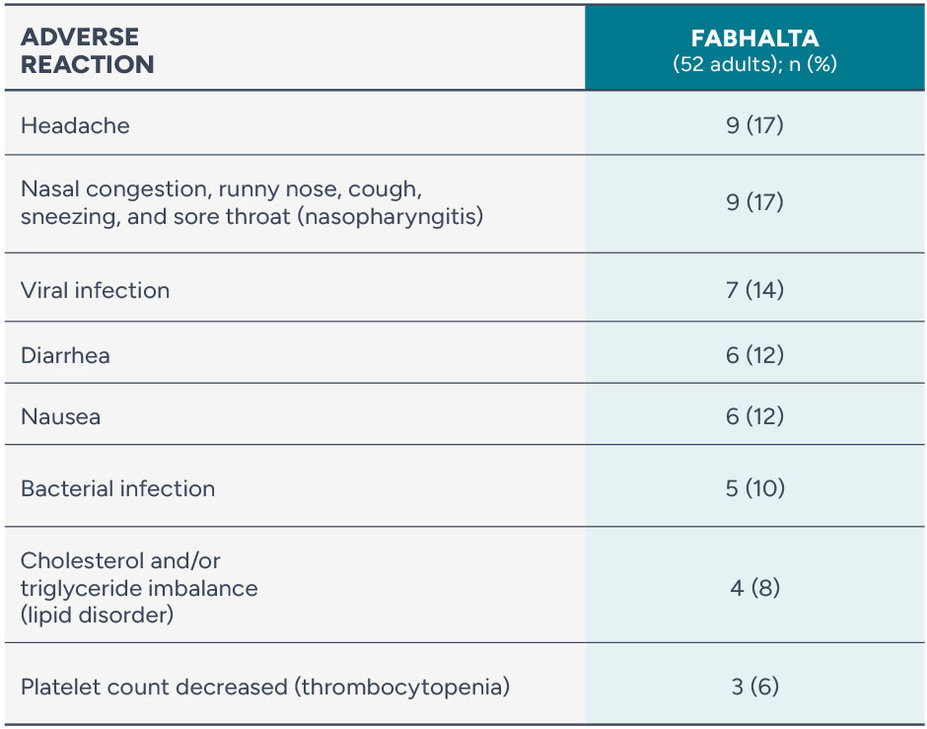
Adverse reactions reported in >5% of adults with PNH treated with FABHALTA (24-week treatment period)
A serious adverse reaction (bacterial pneumonia) was reported in 1 patient (2%).
One person discontinued FABHALTA during the study due to an adverse reaction (palpitations).
ULTOMIRIS (ravulizumab-cwvz) and SOLIRIS (eculizumab) are registered trademarks of Alexion Pharmaceuticals, Inc.